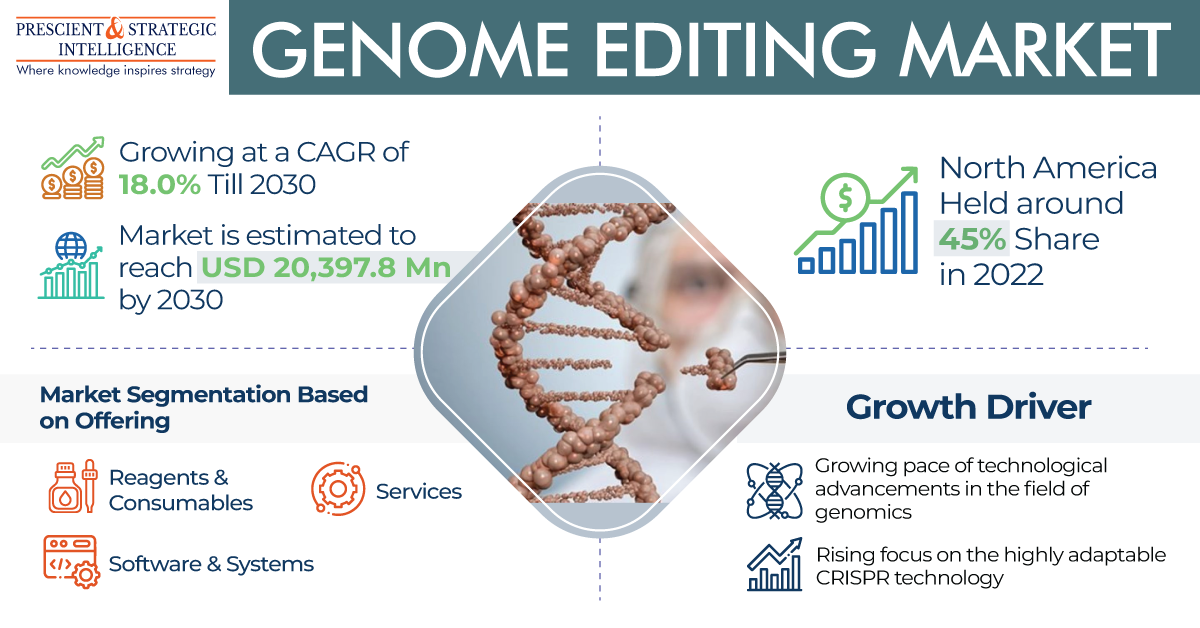
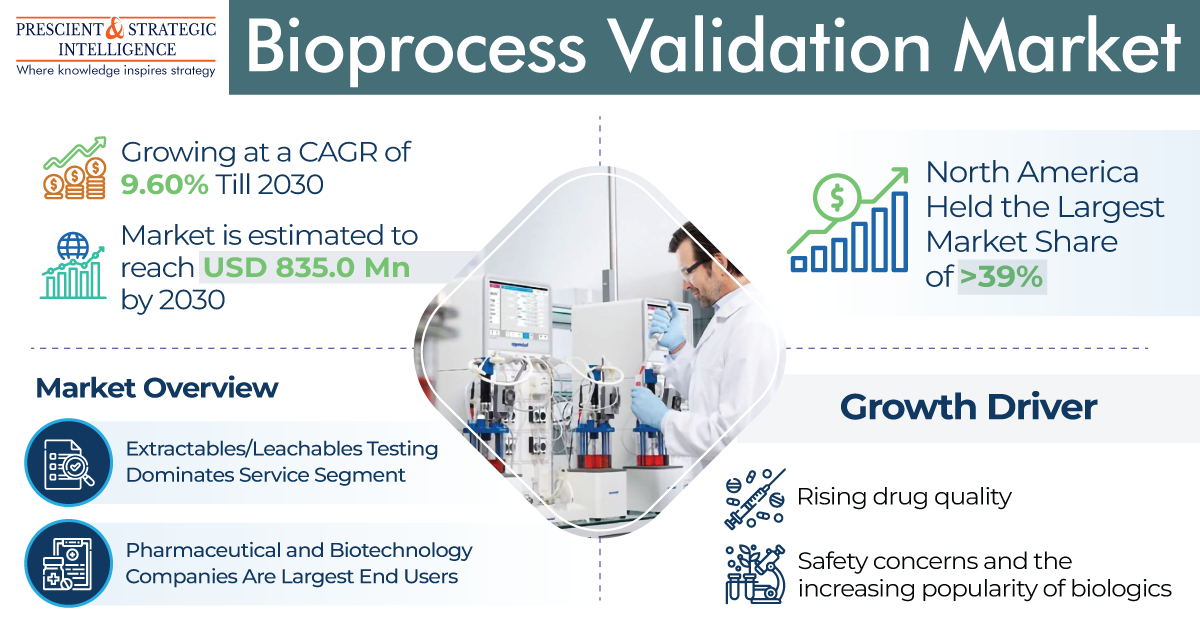
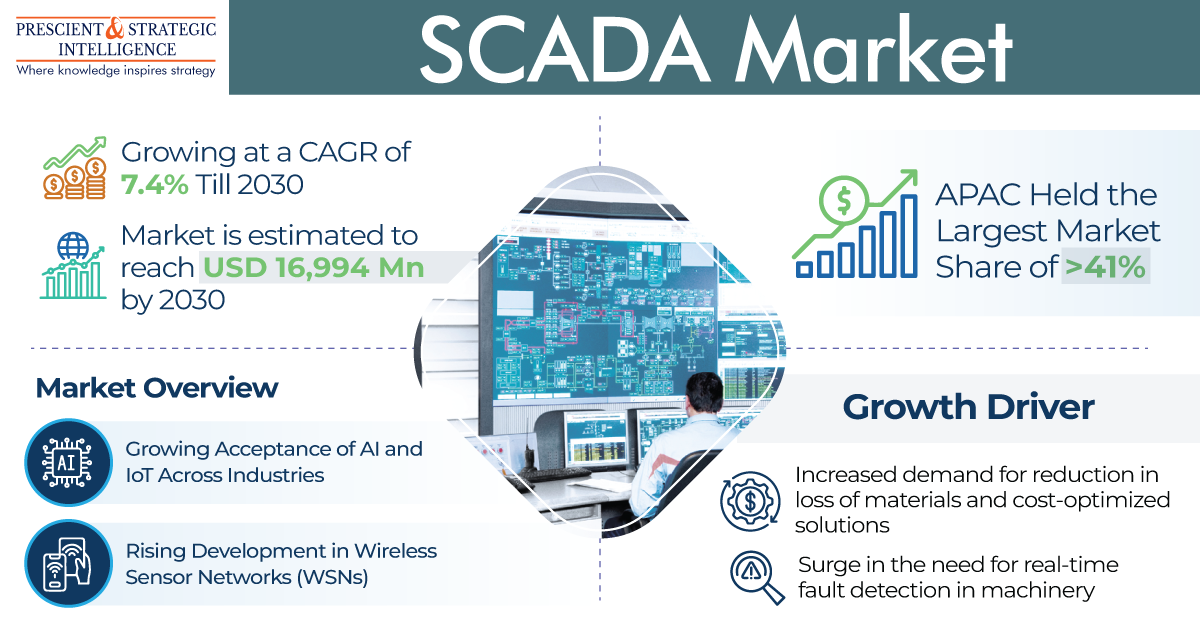
The medical X-ray film processor market, valued at USD 1,024.7 million in 2024, is expected to grow to USD 1,238.5 million by 2030, with a compound annual growth rate of 3.2%.
This growth is driven by the increasing prevalence of cancer, dental issues, cardiac conditions, and orthopedic ailments such as arthritis, alongside the rising number of X-ray imaging procedures, growing healthcare expenditures, and heightened awareness of early diagnosis.
In 2021, around 1.2 million heart surgeries were performed globally. Similarly, approximately 850,000 knee replacements are conducted annually in the U.S., reflecting the growing need for X-ray systems for accurate diagnosis due to the increasing incidence of cardiovascular and orthopedic diseases worldwide.
X-ray imaging has significantly improved the early diagnosis and treatment of various conditions, including tumors, bone injuries, pneumonia, and dental problems. This has led to widespread adoption of X-ray imaging systems and, consequently, X-ray films and film processors. As the incidence of these and other ailments rises, the demand for X-ray imaging for diagnosis continues to grow.
In 2023, the fully automatic film processor segment held the largest market share, at 60%, due to its high image quality. The orthopedic segment dominated the market based on application, attributed to the extensive use of X-rays in diagnosing and examining joint or bone conditions such as dislocation, arthritis, and fractures.
The diagnostic centers segment is projected to experience the fastest CAGR in the coming years, driven by the increasing number of diagnostic centers, their accessibility, and their cost-effectiveness compared to full-fledged hospitals.
North America was the largest contributor to the market, with the increasing prevalence of chronic diseases and the growing elderly population boosting the demand for X-ray imaging procedures. The presence of key players, availability of advanced products, and growing awareness of early disease diagnosis are also supporting regional market growth.
The Asia-Pacific region is expected to grow at the fastest rate in the coming years due to rising R&D activities, government initiatives promoting effective and early diagnosis and treatment, improved healthcare infrastructure, increasing demand for advanced medical imaging devices, and a large patient pool.
The rising prevalence of cancer will continue to drive the growth of the medical X-ray film processor market in the future.