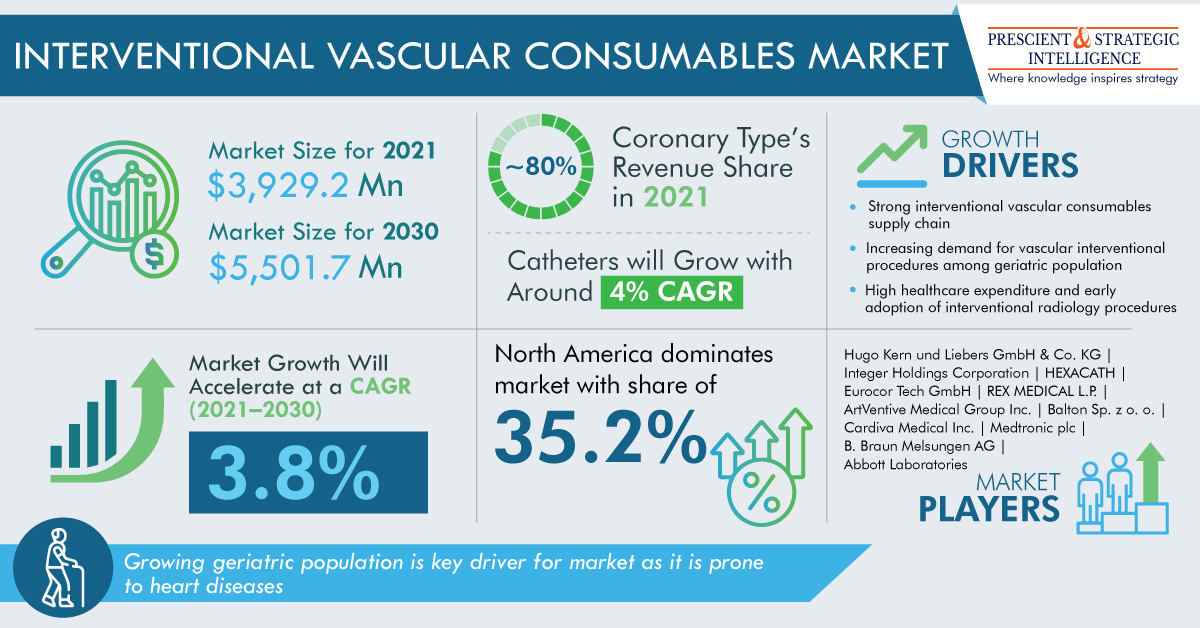
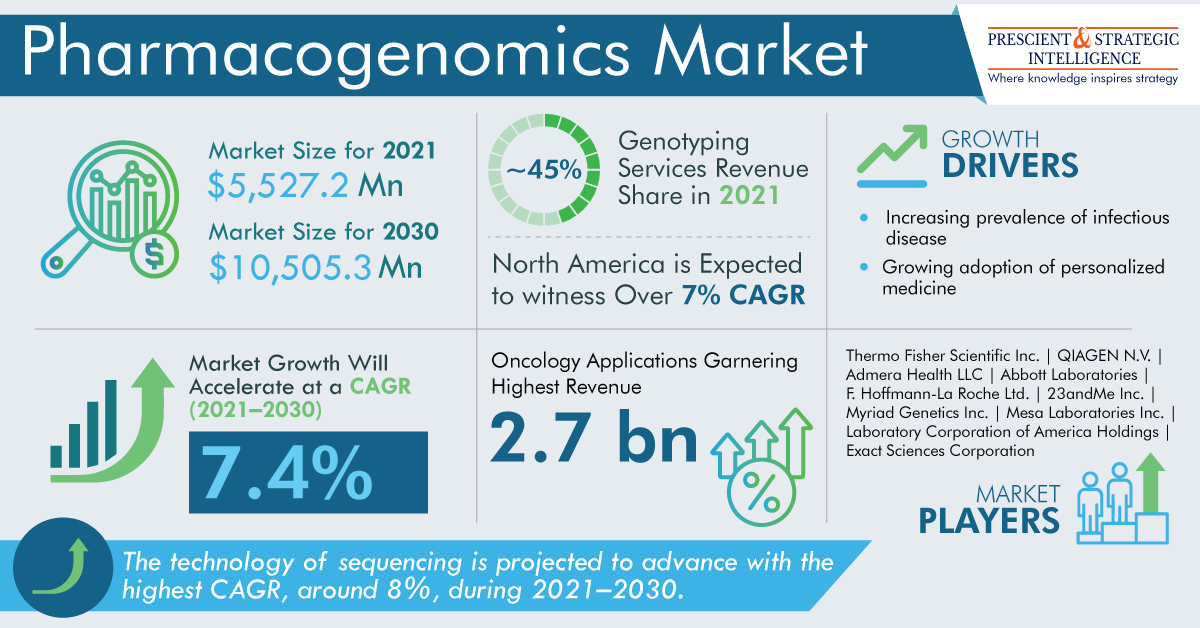
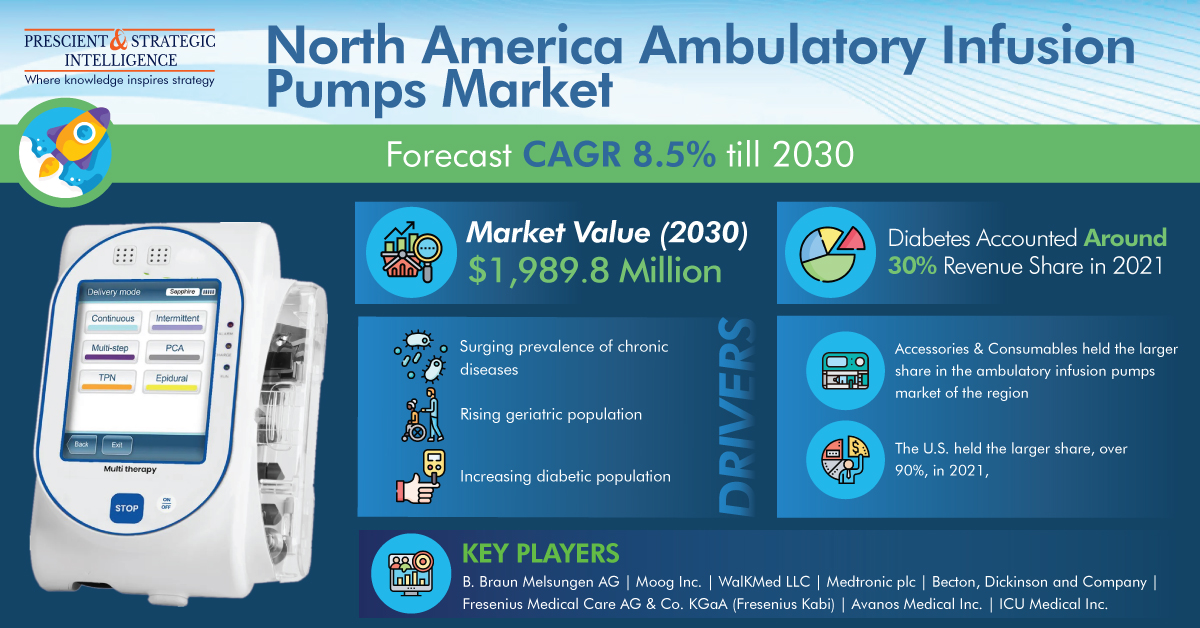
As per a market research report by P&S Intelligence, professional aesthetic lasers market will grow at a rate of 10.3% in the years to come, to reach USD 3,387.4 million by 2030.
This growth has a lot to do with the growing consciousness through conferences and seminars, increasing elderly population, increasing income, budding medical tourism sector, growing volume of aesthetic procedures, tech progression, and surging consciousness pertaining to the safety and advantages of laser-based aesthetic devices.
The skin rejuvenation category had the largest share, and it will retain its position in the years to come. This is because of the increasing incidence of some unwanted changes, for example, scars, fine lines, wrinkles, rough texture, enlarged pores, and dull tone, which become obvious on the human face with age.
North America dominated the professional aesthetic lasers market in the recent past. This has a lot to do with the rising healthcare spending, occurrence of more than a few market players, obtainability of cutting-edge aesthetic laser systems, and increasing consciousness about aesthetic treatments.
Worldwide, more than a few exhibitions, conferences, and seminars are being organized for raising consciousness amongst plastic surgeons, students and general public pertaining to the progressions in anti-aging treatments. More than a few market players are, consequently, aggressively taking part in these exhibitions and events to showcase and introduce their offerings.
The loss of fibrous tissue and decrease in skin stem cells, leading to the incidence of wrinkles, pigmentary alteration, dryness, and sagging, accompanied by the increase in elderly population.
As per UNDESA, the population of people aged 65 years and more will touch 225.4 million in India, 356.6 million in China, 84.8 million in the U.S., and 52 million in Brazil, by 2050.
Due to the increasing elderly population all over the world, the demand for professional aesthetic lasers will continue to grow in the years to come.