Maintaining high-quality standards is crucial throughout the bioproduct manufacturing process. This involves eliminating contaminants and impurities from chromatographic media, which can consist of viruses, nucleic acids, endotoxins, cell membranes, aggregates, culture media components, proteins, ligands, product alterations, process chemicals, and inactive microbes.
To ensure the effectiveness of equipment and methods used in manufacturing processes, rigorous scientific validation exercises with thorough documentation are necessary. The FDA’s General Principles of Process Validation guidelines require documented evidence at all stages of bioprocess validation studies to provide a high level of confidence in the intended product quality for consumers.
Bioprocess validation is an integrated process that adheres to FDA, EMA, and other national and international regulations and standards. It aims to verify procedures and ensure compliance with current Good Manufacturing Practices (cGMP). Extensive documentation is required by national and international guidelines to confirm that the process follows standard procedures.
To improve production many drug manufacturers are turning to third-party service providers. The use of disposable technologies during medication development further reduces production costs. Single-use bioreactors have increased process adaptability and significantly reduced the risk of cross-contamination. These advancements benefit the bioprocess validation sector by shortening commercialization timelines and ensuring product reliability.
The COVID-19 pandemic has also had a significant impact on bioprocess validation. The demand for validation services increased during the pandemic as validation became necessary at all stages of drug development to monitor accuracy, safety, and efficiency. Extensive testing and manufacturing of vaccines drove the need for process validation.
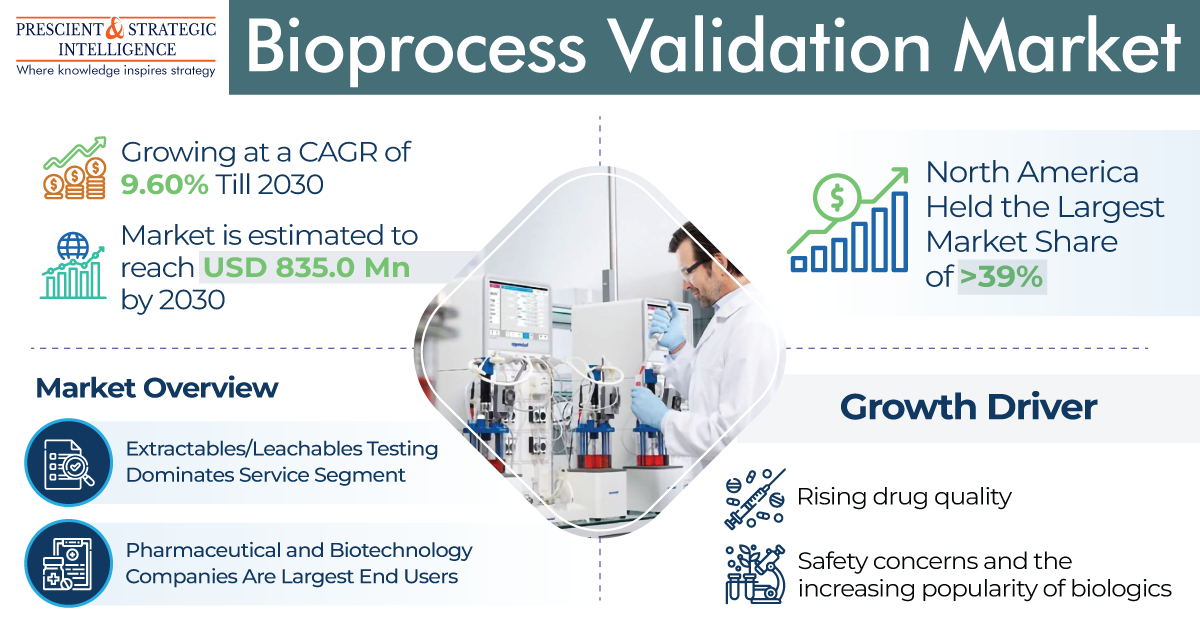
Increasing Focus on Extractables/Leachables Testing Services
Bioprocess validation services include extractables/leachables testing, bioprocess residual testing, viral clearance testing, and filtration and fermentation systems testing. Extractables/leachables testing dominates this service segment due to the rising risk of product adulteration and the presence of regulatory guidelines. Prominent guidelines in this area include those from the FDA and current good manufacturing practice guidelines.
Rising Utilization of Filter Elements
Bioprocess validation products encompass filter elements, media containers and bags, freezing and thawing process bags, mixing systems, and bioreactor transfer systems. The usage of filter elements is expected to significantly increase, primarily driven by their essential role in various bioprocessing techniques to ensure the efficacy and safety of biopharmaceutical products at each production step.
The use of bioreactors for manufacturing vaccines, medicines, and other pharmaceutical components is also on the rise. Bioreactors are widely employed in healthcare research for converting in-vitro 2D culture models and suspensions into the 3D form, replicating the natural physiological state at the site.
Growing Importance of Biopharmaceuticals
The increasing prevalence of chronic diseases is driving the demand for biopharmaceuticals, leading to the rapid adoption of bioprocess validation services across the healthcare sector. Infectious diseases, cancer, and neurological disorders are becoming more prevalent, highlighting the significance of biopharmaceuticals.
Many biopharmaceuticals used to treat neurodegenerative disorders are directly administered into the brain, which is not feasible with conventional synthetic agents. Furthermore, advancements in bio-based drugs have resulted in higher survival rates for cancer patients, hepatitis cures, improved arthritis management, and transformed treatment options for various diseases. The rising incidence of such diseases drives the growth in biopharmaceutical use, thereby boosting the development of the bioprocess validation sector.
The bioprocess validation sector in Asia is advancing significantly due to increased government support for pharmaceutical research and development, a growing elderly population, and a rising trend of outsourcing in the region. Moreover, the global bioprocess validation industry is projected to reach a value of USD 835 million by the end of this decade.